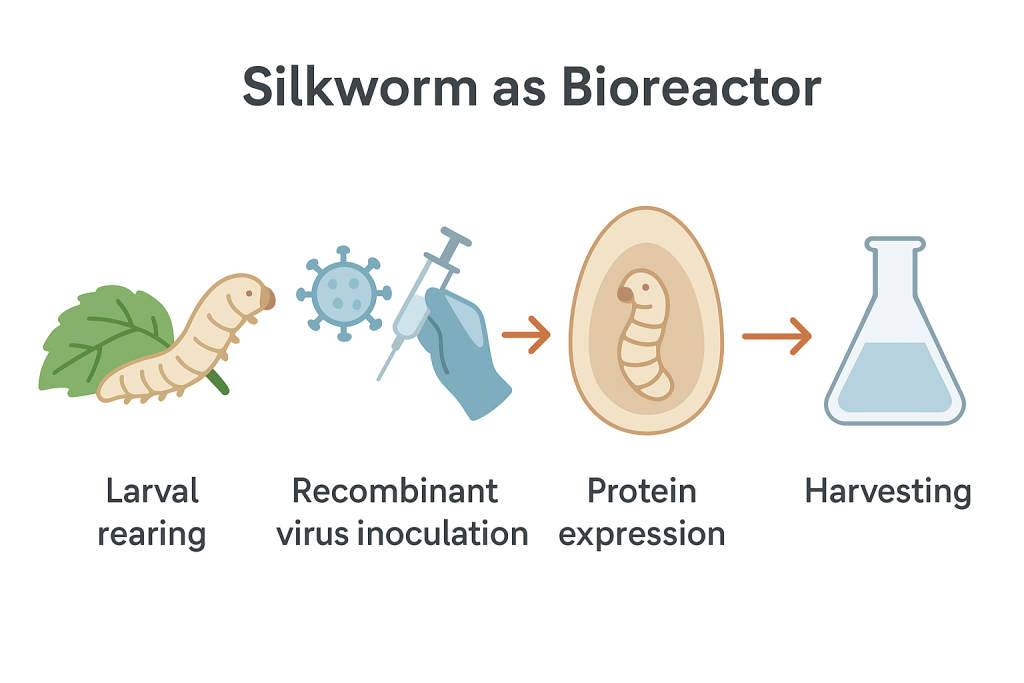
Biotechnology is experiencing a subtle yet profound shift. For years, the creation of antibodies and therapeutic proteins has depended on vertebrate-based systems—most notably mice, rabbits and mammalian cell lines such as CHO. These platforms have enabled major medical breakthroughs, but they come with challenges: high running costs, complex infrastructure, lengthy production cycles and significant ethical considerations.
Increasingly, invertebrates—especially the silkworm Bombyx mori—are offering a viable, efficient and ethically favourable alternative. Below, we explore how this transition is unfolding and why silkworm bioproduction is gaining global momentum.
Why Invertebrates Are Gaining Ground
Ethical and regulatory benefits
In many regions, invertebrates are treated differently from vertebrates with respect to animal welfare frameworks. Their simpler nervous systems equate to fewer ethical barriers and better alignment with global “3Rs” principles aimed at reducing animal use.
Lower production costs
Invertebrate rearing requires a fraction of the investment needed for mammalian cell culture. Silkworms thrive on low-cost diets and simple environmental controls, dramatically lowering operational overhead.
Scalability and rapid output
Each larva or pupa functions as a miniature bioreactor. Scaling up is as straightforward as increasing the number of silkworms—no new facilities, fermentation tanks or high-spec incubators required.
Robust post-translational modification
Unlike microbes, invertebrates can carry out complex folding and glycosylation. While their glycan patterns differ from mammalian equivalents, silkworms reliably produce correctly folded, functional proteins suitable for research and some therapeutic pathways.
Why Silkworms Stand Out
Established global sericulture infrastructure
Centuries of silk production mean rearing techniques, supply chains and husbandry practices are already highly refined. Biotech firms can plug into this ecosystem rather than building one from scratch.
High yields of complex proteins
Using recombinant baculovirus systems or transgenic lines, silkworms produce impressive volumes of functional antibodies and proteins—often comparable to insect cell culture.
Fast development cycles
The timeline from gene insertion to harvest is measured in weeks rather than months. This makes silkworms ideal for emergency vaccine projects, prototype antibody generation and rapid research-scale work.
Consistent glycosylation and folding
Proteins produced in silkworms retain functional structure, with glycan profiles that support antigen binding and stability. Notably, quality remains steady even when rearing conditions vary.
Lower contamination levels
Silkworms naturally produce fewer interfering proteins, simplifying downstream purification. Some studies report contaminant levels as low as 10 parts per million—an excellent starting point for further refinement.
Minimal infrastructure requirements
Silkworm pupae can be stored and handled without dedicated incubators or costly environmental chambers. This lowers the barrier to entry for smaller labs or emerging biotech markets.
A Global Trend: The Invertebrate Biotech Revolution
-
- Japan is leading commercial adoption, with companies such as IBL Japan, Seteka and KAICO producing diagnostic antibodies, vaccine candidates and virus-like particles using transgenic silkworm platforms.
-
- Europe is accelerating research, driven partly by strong ethical and regulatory motivations to reduce vertebrate use.
-
- China and India, long-standing centres of sericulture, are leveraging their expertise to advance silkworm-based bioproduction and are poised to become major global players.
-
- Academic interest is expanding rapidly, with growing evidence supporting invertebrate glycosylation accuracy, scalability and viability for broad biomanufacturing applications.
Together, these developments reflect a shift towards more flexible, sustainable and ethically aligned production technologies.
Limitations to Consider
Silkworm bioproduction isn’t without challenges:
-
- Glycan structures differ from mammalian systems, which may limit suitability for certain therapeutic functions.
-
- Regulatory pathways for invertebrate-derived therapeutics are still developing.
-
- Pharmaceutical-grade production requires rigorous purification and quality assurance workflows.
However, advances in genetic engineering, rearing technologies and transgenic refinement are steadily overcoming these hurdles.
Conclusion
Silkworms and other invertebrates are quickly becoming essential tools in modern biomanufacturing. With lower costs, faster turnaround times, ethical advantages and the ability to produce complex proteins at high fidelity, they represent one of the most promising shifts in the biotechnology landscape.
As global interest grows—particularly in countries that already possess deep sericulture expertise—the humble Bombyx mori is emerging not just as a source of silk, but as a pivotal bioreactor helping shape the next era of diagnostics, therapeutics and research innovation.
Key Points
-
- Invertebrates offer ethical, scalable and cost-effective alternatives to traditional mammalian production systems.
-
- Silkworms excel due to established rearing infrastructure, strong yields and reliable protein folding.
-
- Global interest is accelerating, with Japan, Europe, China and India driving adoption.
-
- Challenges remain but are rapidly being addressed through advances in genetic and rearing technologies.
Silkworm bioreactors are moving from niche research tools to mainstream biomanufacturing platforms. Their speed, affordability and ethical advantages make them an increasingly attractive choice for antibody and protein production, positioning them as a major force in the future of biotechnology.